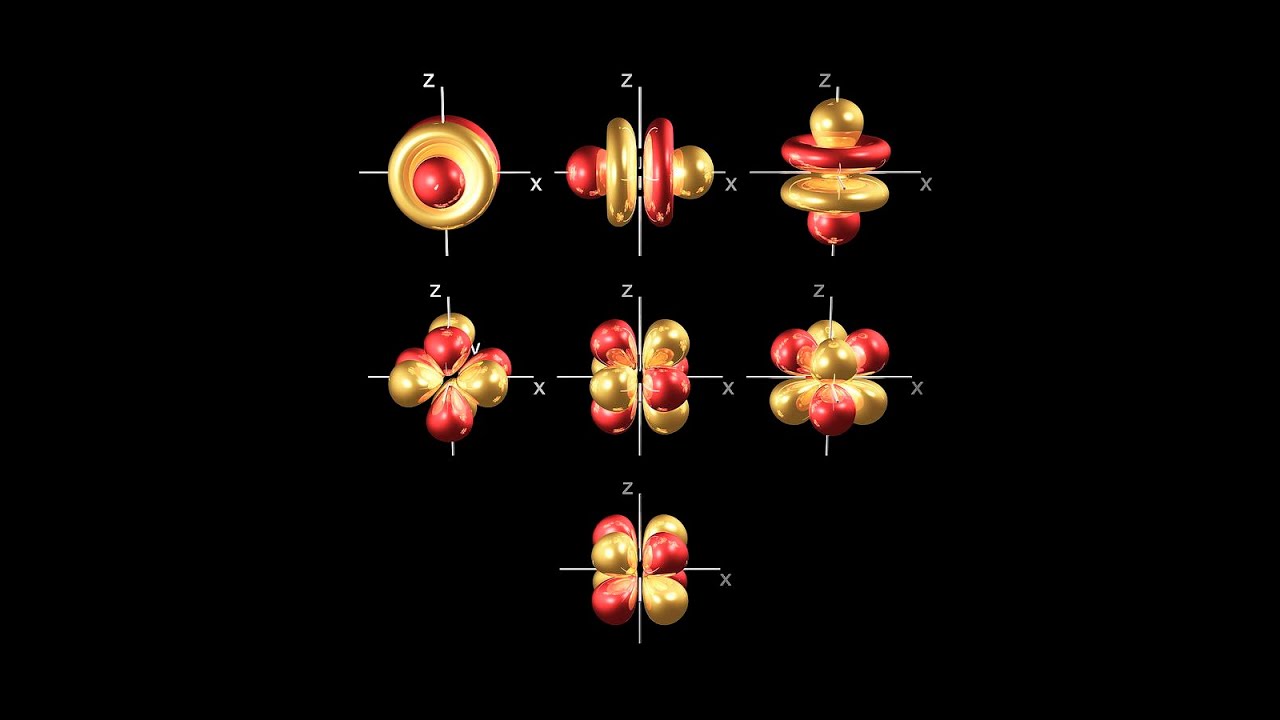The Nature of the Chemical Bond
Guests: Doug Sharp and Rich Geer
Description: Understanding basic chemistry leads to solutions to two important issues for creation scientists. It helps understand how leaching may occur in radioisotope sample causing dates to be skewed dramatically toward deep time. It also helps us to understand how bonding of various types leads to the processes we observe in life. Each element had a nucleus of protons and neutrons, and that nucleus is surrounded by electrons. Electrons have four types of orbitals: s, p, d and f. The s orbital is the simplest in that it is spherical. The other three types of orbitals are standing waves of orbits because the path of the orbits follow the wave function described by Erwin Schroedinger. The parent elements of every one of the elements involved in radioactive decay form ions that are much more likely to dissolve in water than those of the daughter elements. Therefore leaching is one of the more likely explanations why radioisotope dating yields deep time dates. Also, understanding ionic, covalent, hydrophobic, hydrogen and Vanderwaals bonds helps us know how the chemistry of life works.